



Lumpy Skin Disease: Sample collection and shipping
Learn more about lumpy skin disease (LSD)Editors note: The following content is an excerpt from Lumpy Skin Disease: a field manual for veterinarians which is designed to enhance awareness of lumpy skin disease and to provide guidance on early detection and diagnosis for private and official veterinary professionals (in the field and in slaughterhouses), veterinary paraprofessionals and laboratory diagnosticians.
The sampling team should bring sufficient quantities of materials and equipment (see Box 1) for the number of animals to be sampled, plus a margin for materials that may be damaged or become unusable for other reasons (e.g. vacutainers that lose vacuum, etc.). Additionally, items for data collection, personal protection/biosecurity, and transport of samples must be packed. It is recommended to go with a field sampling form so that all needed samples and related information can be collected on-site.
If submission of samples to a regional/international reference laboratory is foreseen, it is advisable to duplicate samples so that one set can be submitted while the other is safely stored. Samples should be taken with care, using appropriate techniques, to avoid undue stress or injury to animals or harm to the sampler. Those in charge of sampling (and of conducting clinical inspections) should previously have been trained in techniques for restraining cattle (both for clinical inspection and sampling).
All samples that have not yet been tested should be considered infected and handled accordingly. All sampling materials used on farms should be disposed of safely and according to local regulations, e.g. bagged and transported back to the laboratory for autoclaving/appropriate disposal. Diagnostic laboratories require the submission of suitable samples that are clearly and permanently labelled and that arrive at the laboratory in good condition.
Preferred sample types
Skin lesions and scabs, saliva or nasal swabs, EDTA blood for PCR assay, whole blood for serum samples.
General rules
Due to the highly characteristic clinical signs of LSD, it is not a common practice to carry out postmortem examinations in the field. Animals presenting a mild case of the disease do not usually have internal lesions, and there is no need to open severely diseased animals as their external lesions are so obvious. The indications listed below therefore refer to sampling from live animals.
- Use protective clothing.
- Restrain or sedate the animals in order to avoid stress or injury, and danger to the operators.
- Work aseptically, avoiding cross-contamination between samples; disinfect the sample collection site, change needles, scalpels, and gloves.
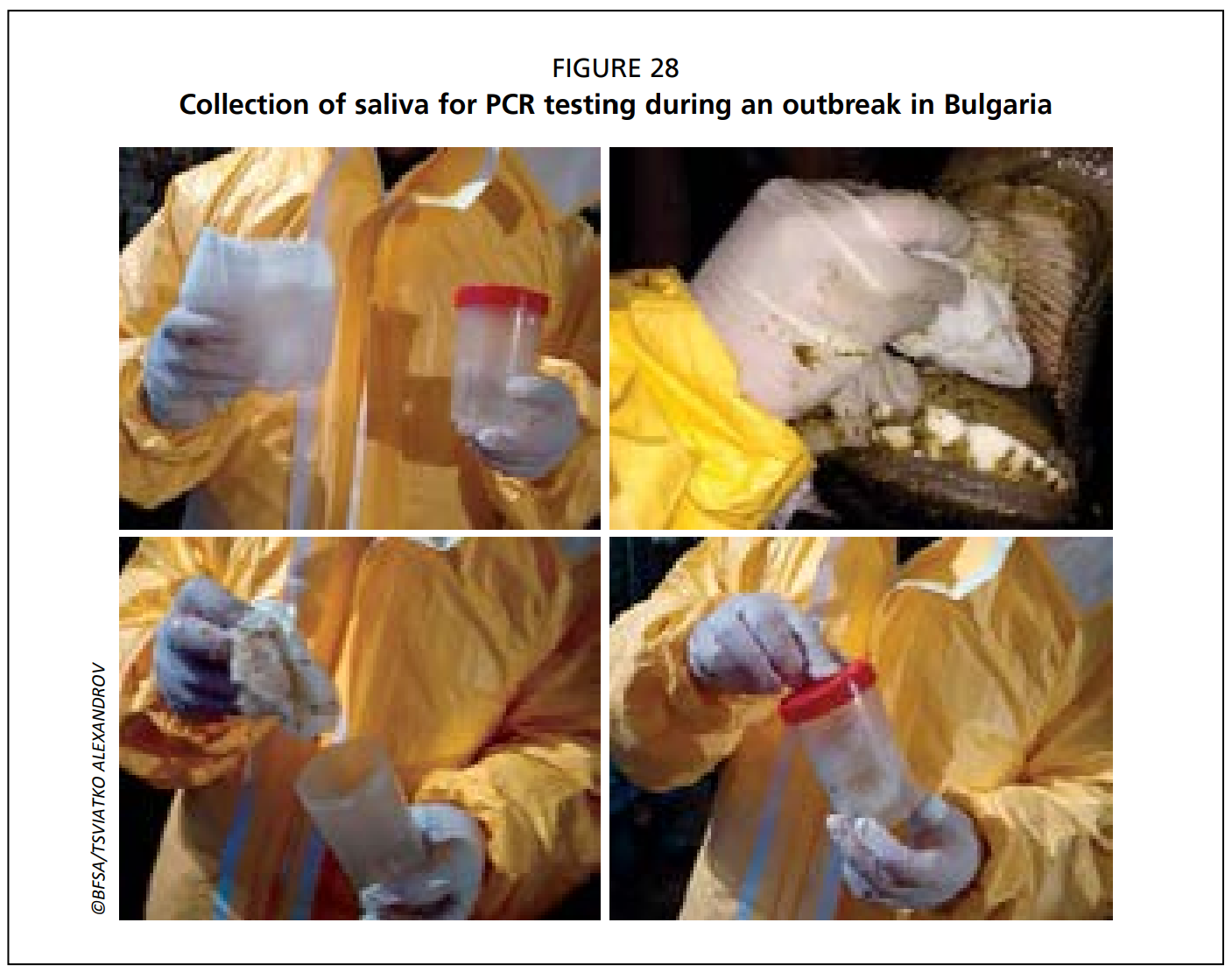
- Saliva and nasal swabs are collected using sterile swabs and placed into sterile tubes for transportation, with or without transport medium (Fig. 28).

- Use local ring block-anaesthesia if you surgically collect full-thickness samples from skin lesions; disposable biopsy punches 16 to 17 mm in diameter can be used.
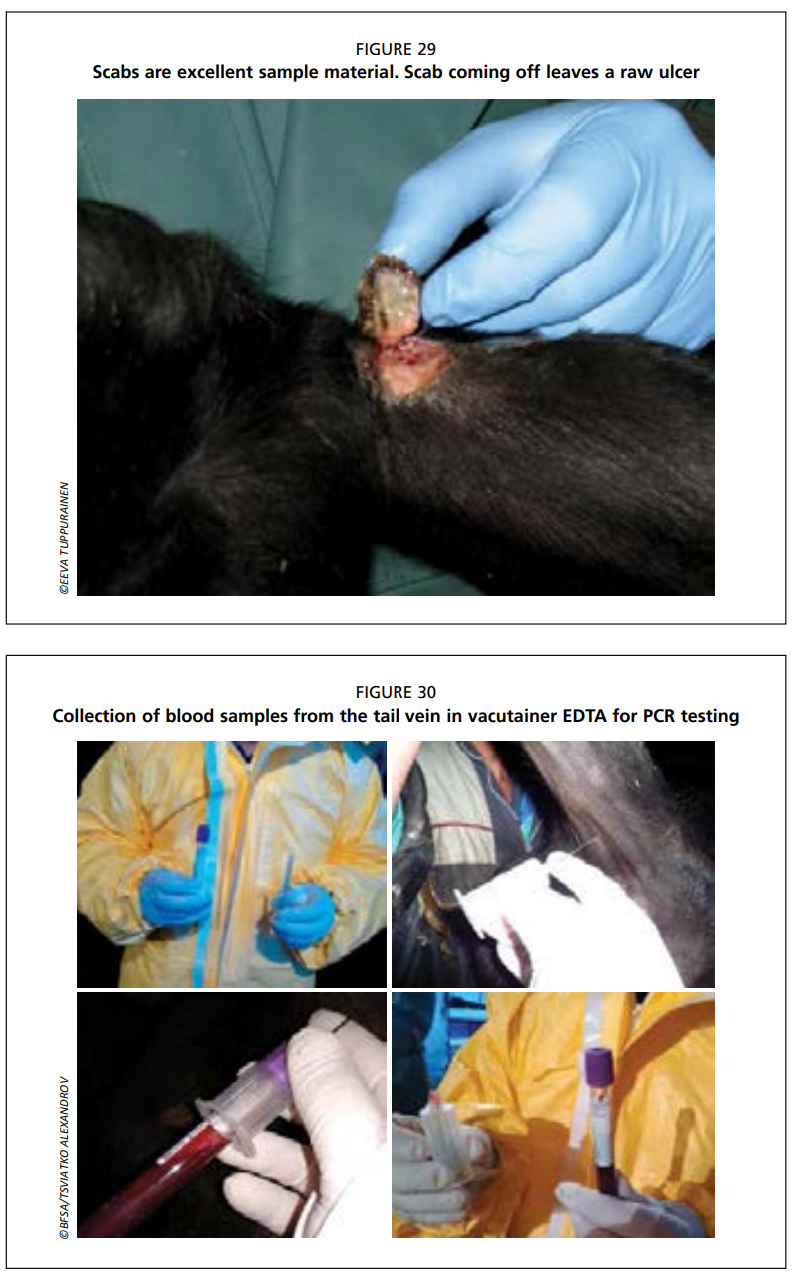
- Scabs are excellent sample material as they are easy to collect, do not require sedation of the animal or local anaesthesia, survive long transport well in different temperatures and contain high concentrations of virus (Fig. 29).
- Collect blood samples from the jugular or tail vein (coccygeal vein).
- Collect sufficient volumes of blood: a minimum of 4 ml of vacutainer EDTA (purple top) is needed for PCR testing (note: heparin may hamper the PCR reaction) (Fig. 30). Tubes without an anticoagulant are used to collect serum samples. The tubes should be filled completely.
- After collection, tubes without anticoagulant should be allowed to stand at ambient temperature for at least 1-2 hours in an upright position to let the clot begin to contract. The clot can then be removed using a sterile rod and tubes stored at 4 °C for 12 hours. The serum can be removed with a pipette or decanted into fresh tubes. If it is necessary to clear the serum, the samples can be centrifuged at slow speed (1000 g/2000 rmp) for 15 minutes, after which serum can be removed. Paired serum samples can be collected 7-14 days apart.
Transport of samples nationally and internationally
Diagnosing LSD is urgent and, to diagnose a disease correctly, it is essential that the right samples are selected, carefully labelled, packaged, and sent to the laboratory at the right temperature, using the fastest practical means of transport by the most direct route. Samples must be accompanied by a submission form. The minimum amount of information required varies depending on the laboratory. It helps to phone the laboratory prior to sampling to ensure that submission procedures are followed correctly and the envisioned number of samples can be analysed or stored in good time.
In general, submission forms should contain the following information:
- number and type of samples and the source species;
- sample ID (one must be able to cross-reference each sample to the source animal);
- owner, name of farm, type of farming system;
- sampling location (address, county, district, province, country of origin, as appropriate);
- name of the person submitting the sample;
- name(s) of the person(s) to whom results are being sent;
- tests required;
- observed clinical signs, gross lesions;
- short epidemiological description: morbidity, mortality, number of affected animals, history, animals involved;
- potential differential diagnoses.
Triple packaging should be used even for road transport. Details of the characteristics of triple packaging can be found on pp. 30-31 – International Transport.
Dispatch and storage of samples - national transport
National regulations must be followed when transporting samples to the nearest laboratory, even if samples are transported by veterinary services staff. Samples should reach the testing laboratory as soon as possible to prevent them from
deteriorating and to ensure a reliable result, as well as to prevent the samples and the environment from being contaminated during transport. Shipped samples must be provided with adequate amounts of cooling materials, e.g. ice packs, to prevent deterioration.
Make sure to do the following:
- Fill sample submission form as described above.
- Mark/label the samples individually using a waterproof marker and, if labels are used, ensure that they will remain attached and are suitable for storage at -20-80 °C.
- Samples should be kept cool during transport to the laboratory by using a cool box with ice or freezer blocks.
- Send the samples in a leakproof, preferably triple container, with absorbent inside.
A. Blood, saliva swabs and tissue samples should be kept at 2-6 °C if the shipment is to last less than 48 hours and at -20 °C if it takes more than 48 hours.
B. Serum samples. If transport takes less than five days, the samples can be kept at 2-8 °C degrees in a refrigerator. If more than five days, the clot should be removed and samples stored at -20 °C degrees.
International transport
International transfer of infectious samples is usually expensive and time-consuming. Central veterinary authorities evaluate if samples need to be sent to an international reference laboratory for laboratory confirmation. If that is the case, the national reference laboratory is responsible for organizing the sample transport, usually with a courier specialized in the transfer of dangerous goods. For Europe, the relevant regulation is the European Agreement on the International Carriage of Dangerous Goods by Road (ADR). For other regions, national regulations must be followed.
If none are available, the UN Model Regulations set out in the 2016 OIE Manual for Diagnostic Tests and Vaccines for Terrestrial Animals (Sections 1.1.2 and 1.1.3) should be followed. Potentially LSDV-infected samples are classified as Infectious Substances Class B (Division 6.2) and IATA packing instructions 650 must be followed (UN3373, Category B). It is forbidden to transport infectious substances as either carry-on baggage, checked baggage, or on one’s person. Prior to dispatch of samples, a contact at the reference laboratory must be informed of the shipment and shipment details must be agreed on. An import permit must be obtained from the reference laboratory and included with the sample transfer documents.
The receiving reference laboratory requires the following data:
- flight number/air waybill number;
- courier tracking number;
- date and time of expected arrival at the airport or the lab;
- two contact persons for potential queries, and details of those to whom test results should be sent (name, telephone number, fax number, e-mail address);
- completed sample submission form/cover letter.
The following documents must be enclosed with the sample package in a waterproof envelope, between the secondary and outer packaging, and also taped outside the package:
- import permit of the receiving laboratory;
- submission form/covering letter;
- a list of contents, including the sample type(s), numbers and volumes;
- air waybill;
- pro forma invoice – indicating that the samples are of no commercial value.
In most cases, use of dry ice is required to keep the samples frozen since transport, including customs procedures, usually takes more than five days.
Category B samples need to be transported inside triple containers. The primary (leakproof, water resistant and sterile) container holds the sample. The lid of each sample container must be sealed with adhesive tape or parafilm, and wrapped with absorbent material. Several sealed, wrapped primary containers may be placed in one secondary container. The secondary leakproof container should contain a sufficient amount of absorbent material. It is usually made out of plastic or metal and needs to meet IATA requirements. Dry ice cannot be placed inside the secondary container due to the risk of explosion. Required labels must be affixed to the rigid outer (third) layer, with sufficient cushioning or dry ice inside. The following labels should be attached:
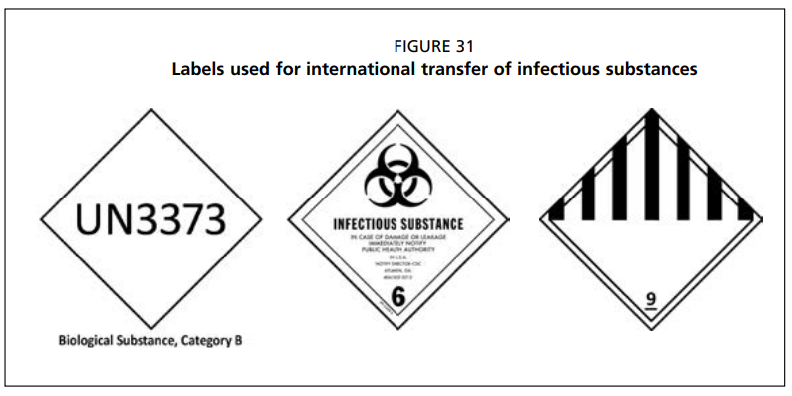
- Infectious Substance/Hazard Label stating that the package contains a “Biological Substance, Category B” Animal Diagnostic Specimen of no Commercial Value (Hazard for Animal Health, not for people);
- Full name, address and telephone number of sender;
- Full name, address and telephone number of addressee;
- Full name and telephone number of a responsible person knowledgeable about the shipment. RESPONSIBLE PERSON: First name LAST NAME, +123 4567 890;
- Label reading “conserve at 4 °C” or “conserve at -70 °C”, as appropriate;
- Label for dry ice (if used) and the proper shipping name of the dry ice followed by the words “AS COOLANT”. The net quantity of dry ice (in kilograms) must be clearly indicated;
- UN number.
Reference:
Tuppurainen, E., Alexandrov, T. & Beltrán-Alcrudo, D. 2017. Lumpy skin disease field manual – A manual for veterinarians. FAO Animal Production and Health Manual No. 20. Rome. Food and Agriculture Organization of the United Nations (FAO). 60 pages.





