



Preventing Drug Residues In Milk and Cull Dairy Cows
By G. M. Jones, Professor of Dairy Science and Extension Dairy Scientist, Milk Quality & Milking Management, Virginia Tech.Preventing drug contamination of milk and meat is the responsibility of every farm. Drug residues can be avoided by a well planned drug use program. There is no way that a milk plant can use contaminated milk. The sale of contaminated milk or meat will cause the responsible party to be subjected to severe penalties, including suspension of permits and monetary loss. Milk with drugs can adulterate a whole truckload or holding tank of milk.
The Food and Drug Administration accepts no drug residue in milk or meat. Sensitive tests can detect a drug from a treated quarter even when this milk has been diluted in the tank by milk from many cows. Milk is checked by the milk plant and by the Office of Dairy Services of the Virginia Department of Agriculture and Consumer Services because:
1. A small percentage of people are violently allergic to antibiotics. Extremely small doses can be fatal. Other people are sensitive to low drug concentrations that cause mild reactions that can be uncomfortable.
2. A continued low-level intake of antibiotics from food could result in a buildup of antibiotic-resistant organisms in humans who are not allergic to the drug.
3. Antibiotics interfere with growth of starter cultures used in making yogurt and cottage or other cheeses.
The major problem with drug residues is the consumers' perception that milk and dairy products, including beef, are pure and free of chemical adulteration or contamination. Consumers want a safe food supply that is free of herbicides, pesticides, and drugs.
The Pasteurized Milk Ordinance requires all Grade "A" milk delivered to dairy plants to be screened for beta lactam antibiotic residues prior to processing. Screening is performed on milk samples obtained from milk tank trucks arriving from farms at milk assembly points. If a tank truck sample tests positive, the sample is retested in duplicate, along with positive and negative control samples, using the same screening method as the original test. At the same time, producer samples from individual farms on the load are tested using the same protocol. The tank truck sample that tested positive, and all producer samples represented on the load, are then subject to confirmation. If the confirmation test is not run, or the confirmation test is positive, the milk from which the representative sample was taken cannot be used for human consumption. Approximately 34% of confirmation tests have been found negative on tank truck samples that were initially screened positive. Of the positive producers, nearly all were traced back to drugs 2. A continued low-level intake of antibiotics from food could result in a buildup of antibiotic-resistant organisms in humans who are not allergic to the drug. 3. Antibiotics interfere with growth of starter cultures used in making yogurt and cottage or other cheeses. The major problem with drug residues is the consumers' perception that milk and dairy products, including beef, are pure and free of chemical adulteration or contamination. Consumers want a safe food supply that is free of herbicides, pesticides, and drugs.
The Pasteurized Milk Ordinance requires all Grade "A" milk delivered to dairy plants to be screened for beta lactam antibiotic residues prior to processing. Screening is performed on milk samples obtained from milk tank trucks arriving from farms at milk assembly points. If a tank truck sample tests positive, the sample is retested in duplicate, along with positive and negative control samples, using the same screening method as the original test. At the same time, producer samples from individual farms on the load are tested using the same protocol. The tank truck sample that tested positive, and all producer samples represented on the load, are then subject to confirmation. If the confirmation test is not run, or the confirmation test is positive, the milk from which the representative sample was taken cannot be used for human consumption. Approximately 34% of confirmation tests have been found negative on tank truck samples that were initially screened positive. Of the positive producers, nearly all were traced back to drugs containing cephapirin or ceftiofur. The majority of violations were caused by failure to observe various provisions of Point 6 of the Milk and Dairy Beef Residue Prevention Protocol/ Administering Drugs And Identifying Treated Animals (Corlett, 1997).
In a survey of farms in the United Kingdom that had bulk milk antibiotic residue violations, the majority of residue occurrences were thought to be lactating intramammary treatments, followed by dry cow mastitis treatments, injections, and intrauterine preparations (McEwen et al, 1991). The three most common reasons were: (1) Failure to withhold milk for the proper length of time, (2) Accidental transfer of milk from treated cows to bulk tanks, and (3) Prolonged excretion of drug from treated cows.
In Michigan, 92.7% of violations were related to mastitis treatments, with 30% involving dry cows (Mellenberger, 1998). A mail survey of Michigan farmers found that most residues were due to: Insufficient knowledge about drug withdrawal periods, Errors due to hired help, Insufficient records of treatment, and Identification of treated animals. Causes of violations are listed in the chart below:
| Causes of violations | |||||
|
Dry cow therapy violations
|
54/179 = 30.2% | ||||
|
Dry cow housed with lactating cows, marked, milked accidentally
|
10.1 | ||||
|
Milk from fresh cow not withheld sufficient time
|
6.7 | ||||
|
Dry cow escaped to milking lot, not marked
|
5.6 | ||||
|
Lactating cow accidentally dry treated, milked
|
3.9 | ||||
|
Cow dry treated, not marked, no one informed milker
|
2.2 | ||||
|
Lactating cows
|
124/179 = 69.3% | ||||
|
Cow marked but milked
|
18.2 | ||||
|
Cow not marked but milked
|
5.8 | ||||
|
Unit attached to cow, removed quickly, tank not tested
|
2.0 | ||||
|
Separated cow, not marked, escaped to lactating group
|
1.0 | ||||
|
Withholding time too short, followed recommended withdrawal time
|
17.0 | ||||
|
Unit not washed after treated cow
|
5.8 | ||||
|
No separate unit to milk treated cows
|
1.6 | ||||
|
Rinse water to flush unit into tank
|
1.0 | ||||
|
Communication failure
|
2.6 | ||||
|
Milk withheld from 1 quarter only
|
1.6 | ||||
|
Purchased treated cows
|
1.0 | ||||
|
Feed (aureomycin crumbles)
|
1.6 | ||||
|
Suggested sabotage (drug in tank)
|
1.6 | ||||
|
Other
|
2.6 | ||||
|
Unexplained
|
7.9 | ||||
The Province of Ontario, Canada, has slightly less than one million dairy cows. In 1988-89, herds with residue violations were visited by field staff of the Ontario Ministry of Agriculture and Food Dairy Inspection Branch. Control herds from the same geographic area were asked to complete a survey. Herds with violations treated more cows for mastitis, and 48% used drugs extra label. Fewer violation herds used separate equipment for milking treated cows and, also, fewer increased withholding times when treatment dosage was increased (McEwen et al, 1991). The authors stated that "the practice of using separate equipment for milking treated cows was unconditionally associated with reduced risk of milk residues and is likely to be more reliable than attempting to divert milk from the tank while using the same equipment for untreated and treated cows alike." Also, they suggested that milking parlors may make it difficult to identify treated cows (or use separate equipment), especially when part-time milkers do the milking.
A total of 809 dairy farms in California, New York, Pennsylvania, Virginia and Wisconsin (160 per state) were asked to complete a survey regarding use of antibiotics and treatment practices in use on their farms (Wilson et al., 1998). Only about half were familiar with the Quality Assurance Program and milkers on only about half of the farms could recognize treated cows. Virginia producers made greater use of written treatment records and on-farm screening tests than producers in these larger dairy states.
|
Producers aware of efforts to reduce residues
|
81.1 |
|
Producers familiar with Milk & Dairy Beef Quality Assurance
|
52.4 |
|
Producers conduct residue tests on farm
|
38.5 |
|
Virginia producers
|
70.6 |
|
Wisconsin producers
|
8.5 |
|
Farms where milkers recognize treated cows
|
5.4 |
|
Virginia farms where: One person treated cows last month
|
42.1 |
|
Two people treated
|
40.7 |
|
Three people treated
|
10.3 |
|
Four people treated
|
3.5 |
|
Virginia farms where treated cows were marked:
|
|
|
All
|
86.8 |
|
51-99%
|
4.2 |
|
1-50%
|
1.4 |
|
None
|
7.6 |
| Farms with written treatment records: | ||||||
| Total | CA | NY | PA | VA | WI | |
| All | 60.0 | 78.5 | 55.3 | 48.6 | 67.9 | 49.3 |
| None | 28.4 | 12..8 | 32.0 | 39.2 | 20.0 | 38.4 |
| 1-50% | 6.5 | 4.7 | 6.7 | 8.2 | 4.3 | 8.6 |
| 51-99% | 5.1 | 4.0 | 6.0 | 4.1 | 7.8 | 3.7 |
Producers were asked for the most likely reason for residue violations and their responses included:
|
% of
Producers |
|
|
Milker being too rushed
|
15.2 |
|
New or different milker
|
10.8 |
|
Lack of communication with milkers
|
4.1 |
|
Not noticing marked cows
|
7.2 |
|
Failure to mark treated cows
|
6.9 |
|
Cow lost identifying mark
|
4.9 |
|
Treated cow rejoining milking herd
|
10.5 |
|
Inadvertent milking of treated cows
|
6.4 |
|
Non-lactating treated cow in milking herd
|
2.6 |
|
Equipment mishap
|
1.9 |
|
Cow aborting or calving prematurely
|
1.0 |
As a result of surveying 219 dairy producers from seven states, Sischo et al (1997) concluded that producers with reported histories of antibiotics in the bulk tank were less likely to implement management changes to reduce risk of an antibiotic residue. The major risk areas identified on these 219 farms included: (1) Treatment records and communication, 53.2%; (2) Understanding how to use antibiotics, 18.0%; (3) Relationship between veterinarians and clients, 15.6%; (4) Use of cowside antibiotic screening tests, 14.4%; and (5) Identification of treated cows, 11.6%.
Guidelines for an Effective Drug Use Program
1. READ THE LABEL when the antibiotic is purchased. It is the responsibility of the dairy producer to understand and FOLLOW DIRECTIONS for usage of all prescriptions and over-the-counter drugs. Make sure that anyone who handles drugs on the farm understands their usage and consequences of misuse. Withdrawal times stated on the label are based on the label-recommended dosage. Off label use probably negates these withdrawal times. Prescription drug labels must include: (1) A cautionary statement restricting use to a licensed veterinarian "CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian." (2) Name of the drug and active ingredients, (3) Withholding or withdrawal times, (4) Name of the manufacturer/distributor or veterinarian, (5) Directions for use, (6) Expiration date, and (7) Dosage level and date dispensed, (8) Any special cautionary statements, and (9) Name and address of the dispensing veterinarian, not just the clinic.2. STORE ALL DRUGS PROPERLY. Drugs must be approved and labeled for use in lactating cows or properly labeled by a veterinarian for "extra label" use. Drugs approved for treatment of non-lactating cattle must be stored separately from drugs used to treat lactating cows and respective shelves identified. Dry cow antibiotics often contain 10 times the dosage used in a lactating treatment. Many drugs require cool storage, and keeping them in a windowsill or on top of the water heater can make the product impotent. At best, they will be ineffective and, at worst, they can promote growth of contaminants that may produce additional problems.
3. ADMINISTER THE DRUG PROPERLY. Always clean the teat end or injection site with alcohol. Be sure to use a sterile cannula if they aren't included with the drug. No medication can do the job right if you inject more germs and contaminants along with the drug. Only insert the cannula tip into the teat by approximately 1/8 inch. After infusing the udder, remember to use teat dip to help sanitize the teat ends against additional bacterial invasion. To avoid new infections from less recognized types of mastitis pathogens (such as pseudomonas, nocardia, yeasts), do not use a treatment cannula on more than one quarter and do not treat several cows from a bottle containing multiple doses.
4. PAY ATTENTION TO WITHDRAWAL TIMES for milk and for cows to be slaughtered. Withdrawal times are not the same for all drugs. If you use a prescription drug from your veterinarian, be sure that you understand the directions for use, withdrawal times, and safe date to market milk or animals treated. Withhold milk from cows treated for intra-uterine infections or other diseases. These drugs can reach the udder and be detected in milk. Do not ship milk or cull cows that may contain drug residues. This includes dry cows if treated at drying off with an intra-mammary product. Wait until the withdrawal time for meat has elapsed.
Milking cows, which are culled because they are unresponsive to mastitis and other treatments, cannot be shipped until the withdrawal time for meat has expired. Remember, carcasses can show needle marks.
If milk from a treated animal is added to the milk tank, don't add any more milk to the tank until the milk has been checked, or dump the milk. Contact the field rep for your milk company.
5. "EXTRA-LABEL" DRUG USE means you have the permission of a veterinarian to use a drug in a way not specified on the label or package insert. When veterinarians authorize giving a higher dose of a drug than is listed on the label or package insert, or using a drug to treat a disease not on the label or package insert, they are prescribing drugs in an "extra-label" manner. It is important to remember that only a veterinarian may use a drug in an "extra-label" manner, and then only with strict limitations. It is illegal for a layman to do so. If cows are treated "extra-label," the withdrawal times must be indicated. Also, it would be prudent to test cows before their milk is added to the tank.
6. MARK AND IDENTIFY ALL TREATED COWS. With expanding herd sizes and different milkers, it is necessary to identify all treated cows and the days which milk must be withheld so they can be recognized by any milker. Separate treated cows from the herd and milk them last as an additional precaution to avoid a tank full of contaminated milk. Accurate observation of the withholding period requires identification at the time of treatment. A good method, which is not infallible, is to double mark cows before treatment with baling twine, neck chains, or special tags over existing numbers; crayon, paint sticks or spray paint, or purple dye on the udder, flanks, legs, or rumps; tape, bailing twine, or plastic bracelets on tails or both legs; or special leg or tail tags which have space for entering the date and time when milk can be saved or cows can be slaughtered. Double marking is good insurance if one marker comes up missing.
7. KEEP A WRITTEN RECORD OF ALL TREATMENTS, including date of treatment, diagnosis or why cow was treated, cow treated, treatment used, withholding times, date when milk can be shipped, and who administered treatment. Although written treatment records were kept on approximately 75% of farms, maintaining some form of written records for determining which cows are being withheld from sale of milk or beef appears to remain an area of opportunity for improvement (Wilson et al., 1998). Provide an easily accessible list of treated cows which shouldn't be milked to milkers at each milking.
8. MILK ALL TREATED COWS LAST OR USE SPECIAL PRECAUTIONS. Segregating treated cows from the rest of the milking herd and milking them last makes it easier to minimize the chance of milking a treated cow. The person doing the milking should have a list of treated cows that he/she verifies before milking begins and checks to see that those cows actually are in that group. Keep TREATED DRY COWS SEPARATE from the milking herd. Keep milk from fresh cows out of the bulk tank for as long as the label recommends.
9. DISCARD MILK FROM ALL FOUR QUARTERS OF A TREATED COW. Drug infused into one quarter can reach all other quarters through the blood stream.
10. ALWAYS REMOVE THE FILL PIPE from the bulk tank prior to placing the milking unit on the first treated cow.
11. DO NOT EXCEED RECOMMENDED DOSAGE LEVELS for Over The Counter drugs and follow veterinarian's directions for prescription or "extra-label" drugs. A double dosage does not double the effectiveness. Administer treatment for as many times as indicated by the directions. If drugs are administered "extra-label," get specific recommendations from your veterinarian regarding treatment, withholding times, and appropriate drug residue tests. Have a treatment plan for cows with mastitis that has been developed and discussed with your veterinarian. Don't treat those cows with mastitis where the chance for success is low, such as those with chronic S. aureus or coliforms.
12. DO NOT COMBINE SEVERAL ANTIBIOTICS YOURSELF. "Home-brewed" concoctions can become contaminated by infectious organisms in the milk house. Withdrawal times would not be known.
13. If medicated feeds are used on the farm, ALWAYS FOLLOW THE FEEDING AND WITH-HOLDING INSTRUCTIONS. Be careful that these feeds do not contaminate the feed or water supply of the milking herd. Make sure that cows do not drink from medicated foot baths.
14. TEST EVERY COW WITH A DRUG RESIDUE TEST before her milk is returned to the bulk tank. Determine whether the residue test used on the farm will detect the drug(s) being used.
15. CARELESS USE OF PESTICIDES AND INSECTICIDES, as well as cleansing and sanitizing agents, can cause contaminating residues in the milk. Be aware of how and where you use them.
16. COMPLETE THE MILK AND DAIRY BEEF QUALITY ASSURANCE PROGRAM MANUAL which is designed to reduce the incidence of violative drug residues in milk and dairy beef by educating producers on proper management/drug usage techniques. Review it with both your veterinarian and your dairy field rep. Do it every year.
DAIRY PRODUCER'S SAFE DRUG USE GUIDE
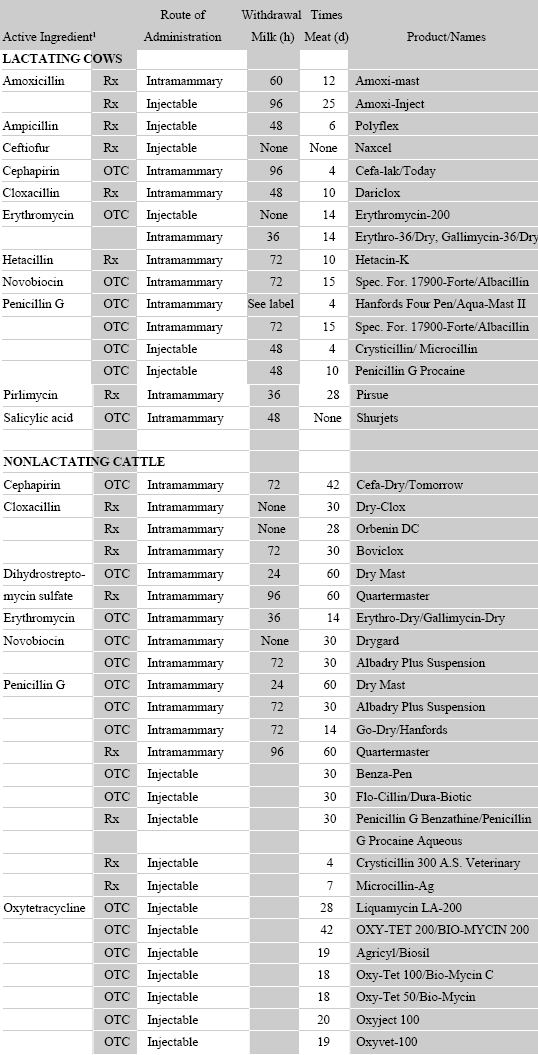
REFERENCES:
Corlett, Jr., N.J. 1997. Findings in field investigations of residue violations. page 186-190 in Proceedings 36th Annual Meeting, National Mastitis Council, Madison, WI.
Dersam, Paul. 1998. A step-by-step method to prevent drug residues. Hoard’s Dairyman, Sept. 10 issue, p.614.
McEwen, S.A., W.D. Black, and A.H. Meek. 1991. Antibiotic residue prevention methods, farm management, and occurrence of antibiotic residues in milk. J. Dairy Sci. 74:2128- 2137.
Mellenburger, R.W. 1998. Milk antibiotic violations: 1996 and 1997 (Mid-March). page 11-14, Michigan Dairy Review, Vol. 3 (1), Michigan State University, East Lansing.
Sischo, W.M., N.E. Kiernan, C.M. Burns, and L.I. Byler. 1997. Implementing a quality assurance program using a risk assessment tool on dairy operations. J. Dairy Sci. 80:777- 787.
Wilson, D.J., P.M. Sears, and L.J. Hutchinson. 1998. Dairy producer attitudes and farm practices used to reduce the likelihood of antibiotic residues in milk and dairy beef: A five state survey. Unpublished data.
May 1999



